A. It’s not good – especially if we’re trying to reach herd immunity by the end of 2021.
Getting “back to normal,” or some semblance of normal, even for the U.S., will require widespread uptake of COVID-19 vaccines all around the world. The virus doesn’t adhere to national boundaries, and long-term travels bans to limit spread are not a realistic solution. Allowing the virus to circulate unchecked also provides a breeding ground for the emergence of variants, as we see happening now. As Dr. Fauci has noted, the best way to stop the spread of COVID-19 and to prevent new variants is to vaccinate as many people as quickly as possible.
➡️So how are countries doing around the world?
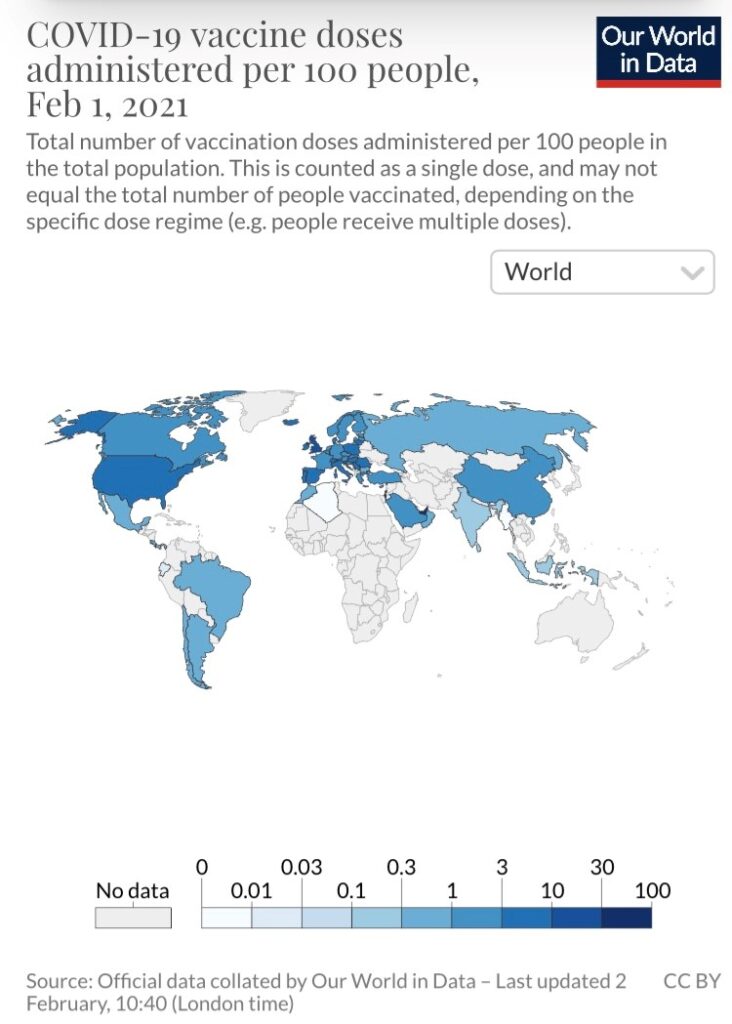
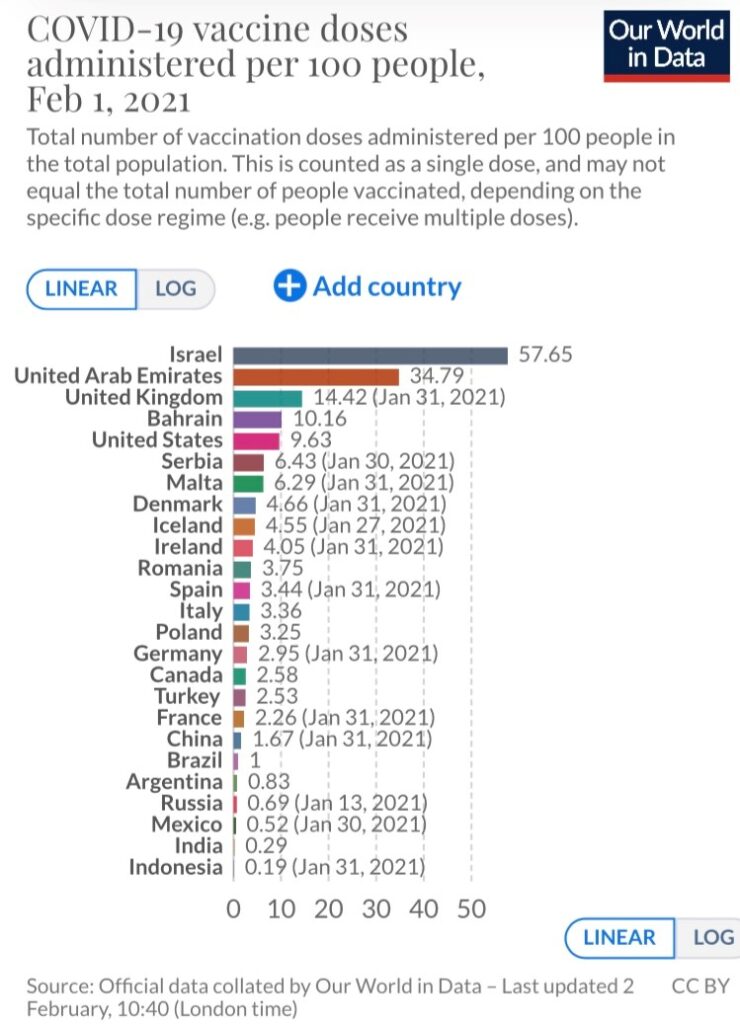
The “Our World in Data” project shows some countries doing a much better job than others at vaccinating their respective populations. For example, Israel currently leads the way, having administered 56.2 doses per 100 people, followed by the United Arab Emirates (35.7 doses per 100 people). As a comparison, the estimate for the U.S. is 9.7 doses.
Except for the United Kingdom (14.7), all European countries trail the U.S. by a considerable margin. At the extreme end of the spectrum are the African continent and Latin America, two locations where concerning strains have emerged (South Africa and Brazil). A majority of the countries in these regions have not administered a single dose. Vaccines have not been administered in Australia or New Zealand either, but these are also countries that have had transmission under control through widespread mitigation measures.
➡️Even among countries that have begun vaccinations, there are differences in the types of vaccines approved for use. The following is a breakdown of the 10 vaccines currently being administered around the world, all with different levels of efficacy, storage requirements, and even Phase 3 trial status (some vaccines were approved for use without adequate Phase 3 trial data).
All require 2 doses:
NOTE: In the U.S., only Pfizer/BioNTech and Moderna have received approval for emergency use. Information below comes from: https://nyti.ms/3rgaEDL.
1️⃣ Pfizer/BioNTech (mRNA):
Efficacy: 95%
Storage requirement: Freezer storage at –70°C
Approved for use in: Bahrain, Saudi Arabia, Switzerland.
Emergency use in: Argentina, Australia, Canada, Chile, Colombia, Costa Rica, Ecuador, European Union, Iraq, Jordan, Kuwait, Lebanon, Malaysia, Mexico, Oman, Panama, Qatar, Serbia, Singapore, Switzerland, Tunisia, United Arab Emirates, United Kingdom, United States.
Emergency use validation from the World Health Organization.
2️⃣ Moderna (mRNA):
Efficacy: 94.5%
Storage requirement: 30 days with refrigeration, 6 months at –20°C
Emergency use in: Canada, European Union, Israel, Switzerland, United Kingdom, United States.
3️⃣ Sputnik V (adenovirus vector)
Efficacy: 91.4% (Phase 3 trial results just published!)
Storage requirement: Freezer
Early use in: Russia.
Emergency use in: Algeria, Argentina, Armenia, Belarus, Bolivia, Guinea, Hungary, Iran, Palestinian Authority, Paraguay, Serbia, Tunisia, Turkmenistan, United Arab Emirates, Venezuela.
4️⃣ Oxford/AstraZeneca (adenovirus vector)
Efficacy: 62% to 90%, depending on dosage
Storage requirement: Refrigeration
Emergency use in: Argentina, Bangladesh, Bhutan, Brazil, Chile, Dominican Republic, El Salvador, European Union, India, Maldives, Mexico, Morocco, Nepal, Pakistan, South Africa, United Kingdom.
5️⃣ Convidecia by CanSino Biologics (adenovirus vector)
Efficacy: unknown (Phase 3 trials ongoing)
Storage requirement: Refrigeration
Limited use in: China.
6️⃣ EpiVacCorona by Russia’s Vector Institute (peptide-based vector)
Efficacy: Unknown (Phase 3 trials ongoing)
Storage requirement: Refrigeration
Early use in: Russia.
7️⃣ Sinopharm with the Beijing Institute (inactivated SARS-CoV-2 virus)
Efficacy: 79.3% (Phase 3 results unpublished)
Storage requirement: Refrigeration
Approved for use in: Bahrain, China, United Arab Emirates.
Emergency use in: Egypt, Hungary, Jordan, Pakistan.
8️⃣ CoronaVac by Sinovac (inactivated SARS-CoV-2 virus)
Efficacy: 50.38% (Phase 3 results unpublished)
Storage requirement: Refrigeration
Emergency use in: Azerbaijan, Brazil, China, Chile, Indonesia, Turkey.
9️⃣ Sinopharm with the Wuhan Institute of Biological Products (inactivated SARS-CoV-2 virus)
Efficacy: Unknown (Phase 3 trials ongoing)
Storage requirement: Refrigeration
Limited use in: China, United Arab Emirates.
🔟 Covaxin by Bharat Biotech in India (inactivated SARS-CoV-2 virus)
Efficacy: Unknown (Phase 3 trials ongoing)
Storage requirement: Room temperature
Emergency use in: India.
******************************************************
CAVEAT: In some countries, certain vaccines are only being recommended for use in some segments of the population. For example, China is only using its vaccines (Sinopharm and Sinovac) among adults < 60 years old until more Phase 3 trial data are available. For the Oxford/AstraZeneca vaccine, because of limited data in older age groups, Germany is considering recommending it only for adults <65 years. Italy has also recommended that the vaccine be prioritized for adults <55 years old.
✳️Clearly a lot more work needs to be done to ensure widespread access to vaccines around the world, especially for high-risk populations, and for countries with limited means to pay for vaccines. We’ll continue to post updates on the international vaccine landscape and we’ll talk more about the successes and challenges on this front in future posts.
Stay safe, stay sane!
Love,
Those Nerdy Girls
LINKS:
Our World in Data COVID-10 Global Vaccination Stats
NY Times Coronavirus Vaccination Tracker