A: It’s been a busy couple of weeks for European and UK vaccine regulators.
TL;DR:
➡️ Investigations continue into a possible link between the Astrazeneca vaccine and a very rare type of blood clot.
➡️ Both the UK and EU regulators continue to emphasize that vaccine prevents COVID-19 disease, and the benefits of the vaccine outweigh the risks.
➡️ The system for reporting vaccine adverse events is working as it should.
➡️ The unusual clotting events are extremely rare and treatable, if they are identified.
➡️ We know the news on Astrazeneca has been somewhat confusing and worrying. The Nerdy Girls are committed to transparency and updating with new info as it emerges—this is the essence of SCIENCE. It also reflects the foundation of trust we hope we have built with this Nerdy community.
Our readers may recall our post couple of weeks ago when several European countries first suspended use of the Astrazeneca vaccine due to reports of blood clots. At the time we reminded followers that it is a common tendency to attribute medical events that happen shortly after vaccination to the vaccine even though many things might happen during that time *just by coincidence.*
Blood clots are fairly common, and the overall rate of blood clots reported out of the millions of Astrazeneca doses distributed at the time was not above what we normally see.
As more information was released, it became clear that the red flags raised in the reporting system were due to cases of an unusual specific type of clotting event in the brain and abdomen—called cerebral venous sinus thrombosis (CVST) and splanchnic vein thrombosis. These were combined with low levels of blood platelets. Out of 25 million doses of Astrazeneca given in the EU and UK, 62 cases of CVST and 24 cases of splanchic vein thrombosis were reported, including (very sadly) 18 deaths.
The European Medicines Association (EMA) reported that most of the cases were in women under the age of 60 within two weeks of the first dose, but we don’t have more specifics on the age and sex breakdown yet.
The cases are still under investigation by hematologists and a definitive link with the vaccine has not yet been established. But because this combination of symptoms is unusual, the reporting system succeeded in flagging above expected levels of this rare type of blood clots. This means that even if a causal link is found, it’s unlikely that every case was linked to the vaccine, as some would have been expected even without vaccination. This is important for quantifying the risks.
Scientists are looking into “biological plausibility” for the link. One plausible explanation for the combination of blood clots and low blood platelets is an immune response, leading to a condition similar to one seen sometimes in patients treated with heparin (heparin induced thrombocytopenia, HIT).
Does the possibility of a direct link between the vaccine and these cases mean use of Astrazeneca will be suspended?
No. These cases are still extremely rare, and if they are identified they can be successfully treated. The risks of COVID-19 disease itself are much higher, especially with increasing age. In fact, the risk of blood clots alone from COVID-19 is much higher!
The EMA reviewed the data and maintained that the benefits of the vaccine outweigh the potential risks with no change in recommendations other than for clinicians to look out for symptoms of this rare events.
Despite the recommendation, several European countries including Germany, Spain and Italy are restricting the Astrazeneca vaccine to those over age 60, age 55 in Belgium. Elsewhere, Australia is restricting use of Astrazeneca to those over 50, while in Canada the age is 55 and up.
The UK Medicines and Health Care Products Regulatory Agency (MHRA) and Joint Commission for Vaccination and Immunization (JCVI) also released a statement concluding that the benefits of vaccination outweigh the risks. But the UK government now recommends that those under the age of 30 be offered an alternative vaccine to Astrazeneca where possible, as a precaution.
How can individuals assess the risks and benefits of taking the Astrazeneca vaccine?
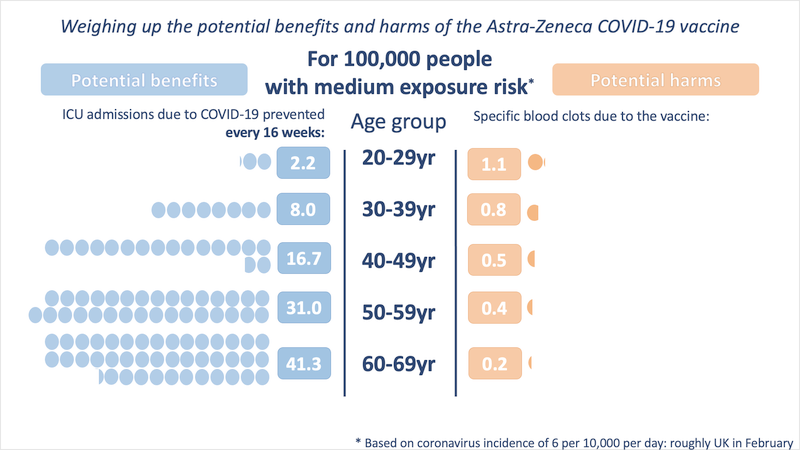
The attached infographic helps visualize the competing risks of COVID-19 vs the risks of the rare events if caused by the vaccine. The UK’s recommendation for people under 30 being offered comes from the risks becoming more comparable at these younger ages. Over age 30, the risks from severe COVID-19 disease quickly dwarf the risks of rare vaccine side effects.
In the full link below you can see these risks compared in a low, medium, and high exposure risk scenario depending on the level of transmission in the community. While case levels are currently low in the UK, they are quite high in many other places in Europe, which makes the benefit of the vaccine even greater.
It’s important to note that the risks of COVID-19 in the figure are quantified as the risk of ICU admission over 16 weeks. So, this doesn’t count additional risks from other hospitalizations or ‘long COVID.’ The risk from COVID-19 also continues, while the risk from the vaccine is limited in time.
For each country and individual, it is also important to consider what alternative vaccines are available. If equally effective vaccines are immediately available that do not carry the risk of a blood clot reaction, utilizing those alternatives could make sense. But if other vaccines are not immediately available, then the risk of exposure to the COVID-19 virus during any delay would outweigh the risk of vaccinating.
Importantly, early treatment can improve outcomes in these rare cases. Patients are advised to seek immediate medical treatment for symptoms including:
• new onset of severe headache, which is getting worse and does not respond to simple painkillers
•an unusual headache which seems worse when lying down or bending over, or may be accompanied by blurred vision, nausea and vomiting, difficulty with speech, weakness, drowsiness or seizures
•new unexplained pinprick bruising or bleeding
•shortness of breath, chest pain, leg swelling or persistent abdominal pain
BOTTOM LINE:
Science, while awesome and the source of these amazing life-saving vaccines, can also be messy. Rare side effects from new medicines and vaccines do occur, and we have surveillance systems in place to identify these– then assess and mitigate the risks.
The deaths from these rare clotting events are real and heartbreaking. However, the vaccine safety monitoring system is working to help flag this and other possible rare side effects. Because the risks of COVID-19 exposure and disease are currently so high and the adverse events so rare, the benefits outweigh the risks for most people.
As NHS Doctor @dresmerelda stated well on Instagram:
“If the AZ vaccine is the one available to you, get it.”
“Refusing it is a bit like not buying a house on a street where one was struck by lightning, and instead, buying a house on a street where several get burgled each week.”
Love,
Those Nerdy Girls
Additional Reading:
Source of Figure with additional risk calculations
“In rare instances, AstraZeneca’s Covid-19 vaccine linked to blood clots, regulators say.”
“AstraZeneca vaccine: How do you weigh up the risks and benefits?”
European Medicines Association statement
Great overview piece by Hilda Bastian: “We Need to Talk About the AstraZeneca Vaccine