A: The best evidence (still) suggests a resounding nay.
***** EDITED: 11:44 am to include FDA evidence review in the reference section *******
For our TGIF post we’re going to revisit the hydroxychloroquine (HCQ) debate, because let’s get real, friends…..it’s been quite the week on the HCQ information frontlines.
We Nerdy Girls are here to help you separate out the JEWELS from the JUNK when it comes to big claims about potential COVID-19 treatments. And today we are going to equip you to think like a Nerdy Girl and join in the infodemic-fighting fun. Welcome to the team!
So how does a Nerdy Girl sort through claims about a (controversial) medical treatment? With kindness, curiosity, and scientific skepticism, of course! And what, exactly, do we mean by “scientific skepticism” when considering evidence about said medical treatment? Enter…… the Three C’s – CONTROL, CHANCE, and CONTEXT. We first introduced the Three C’s in a post discussing the anti-viral Remdesivir (link below), and now we’ll use it to think through this week’s HCQ hot potato. For those who are new to the party (and for those who might welcome a refresher :)), let’s define each of the Three C’s in turn as we apply them to assess the state of the evidence about HCQ:
1. CONTROL: How credible is the comparison between those who get the treatment and those who don’t? Best protection: randomization.
Do we have randomized controlled trials (RCTs) about HCQ? Yes! (see Newsweek reference below for an excellent write-up) What do they find? No evidence of HCQ benefit.
2. CHANCE: How likely were the results to have arisen from the play of chance? Best protection: large sample sizes. “Randomness doesn’t look random” is a common maxim among researchers, and small samples are particularly vulnerable to data flukes that represent statistical noise as opposed to meaningful signal.
Do the randomized controlled trials finding no benefit of HCQ have sufficiently large sample sizes? Yes!
3. CONTEXT: How well do the results translate beyond the research setting? Best protection: Replication across multiple geographies, time periods, and health care
settings.
Has the finding that HCQ confers no benefit been replicated in multiple randomized controlled trials conducted in different settings and at different stages of the COVID-19 disease course? Yes!
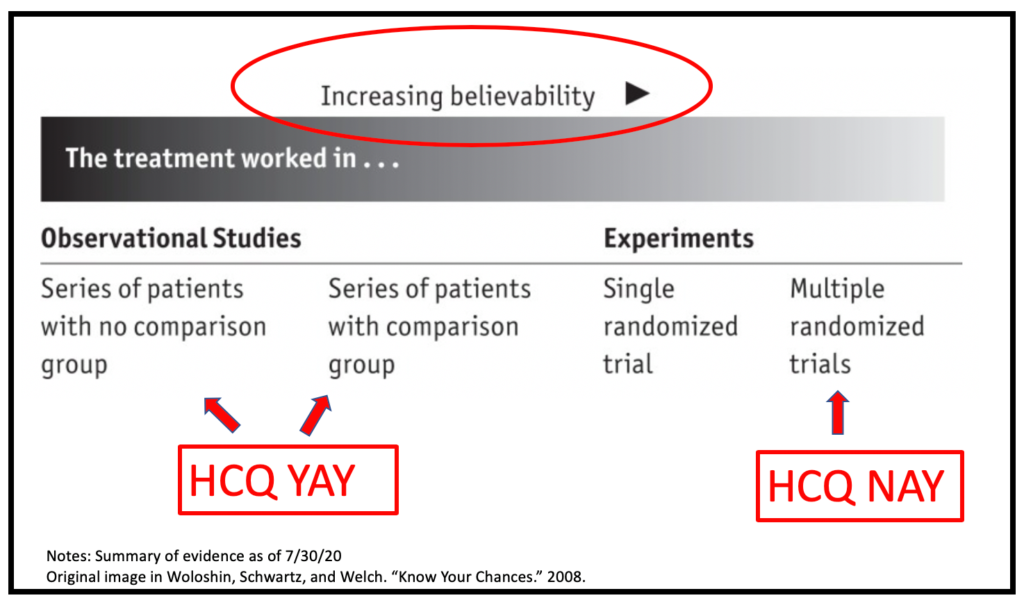
Now here’s your homework assignment (don’t worry, it won’t be graded!): Work through the Three C’s for evidence generated from a handful of (controversial) clinicians’ experiences treating all of their COVID-19 patients with HCQ (Answer: No control group; small enough samples to be subject to the vagaries of chance; poor generalizability across settings). As Fred Guterl astutely describes in the terrific Newsweek article mentioned above, “such claims would be considered just another observational [i.e. non-randomized] study—if s/he were to document it and publish it in a peer-reviewed journal.” You’ll see that this quote jives with the visual depicted below.
And here’s the thing: clinicians practicing evidence-based medicine are the FIRST to incorporate evidence from rigorously-conducted studies performed in a variety of external settings into their clinical decision-making toolkit. Indeed, medical societies demand it.
But you don’t need to take our word for it – or anybody else’s – and we recognize that it’s SO confusing when dueling “experts” with fancy credentials are zinging each other from both sides. We Nerdy Girls will continue to equip you with critical thinking tools like the Three C’s so that you feel more empowered to make your own judgements.
Bottom line: The best evidence (still) suggests a RESOUNDING NAY on the effectiveness of HCQ as a COVID-19 treatment. Researchers continue to work with speed, innovation, and resolve to find other promising treatments – so keep up the hope.
References:
Terrific (!) Newsweek write-up summarizing the history and findings of the key HCQ studies to-date (including a randomized controlled trial demonstrating no evidence of benefit in outpatient settings).
Earlier DP posts on HCQ: Post 1 and Post 2
DP post on Remdesevir/introduction of the Three C’s
Scientific review of the HCQ evidence-to-date in inpatient settings by the FDA
Image adapted from the *FANTASTIC* (and freely available!) book “Know Your Chances,” by Drs. Steven Woloshin, Lisa Schwartz, and Gil Welch. This trio has been at the forefront of fighting “infodemics” for decades, and their work is A-MAZING. You can download the book here.