TODAY IS THE DAY!
The U.S. Food and Drug Administration meets to discuss KIDS vaccines TODAY (October 26th)!
The FDA released summary documents ahead of today’s VRBAC meeting which foreshadowed their assessment of the current evidence for vaccinating 5-11-year-olds.
➡️ TL;DR: The FDA estimates that vaccine benefits “clearly outweigh” the risks for this age group.
While this not the final say (the CDC’s Advisory Committee on Immunization Practice (ACIP) meets November 2nd & 3rd to discuss and make a recommendation), this meeting is an important step in weighing the evidence.
The briefings gave us our first good look at data from the Pfizer/BioNTech pediatric trial, which looked very positive.
⚡Highlights⚡:
Pfizer is using a 10-microgram dose for this age group (compared to 30-microgram for ages 12+) given three weeks apart. This was based on Phase I trial data showing this dose elicited an immune response in 5–11-year-olds similar to a 30-microgram dose in 16-25 year olds, but had fewer side effects than a higher dose.
➡️ The trial evaluated safety and effectiveness.
🧷 Safety was evaluated in two sets of trial participants: 2250 trial participants (including 1518 vaccine recipients) followed for at least two months past the 2nd dose, and a supplemental “safety expansion” group of an additional 2250 participants (1500 vaccines) followed for a median of 2.4 weeks after the second dose.
The reactogenicity (side effects) of the vaccine for this group overall were considered mild to moderate and typically resolved in one to two days. The most common reactions were injection site pain, fatigue, headache, muscle pain, and chills. (See figure)
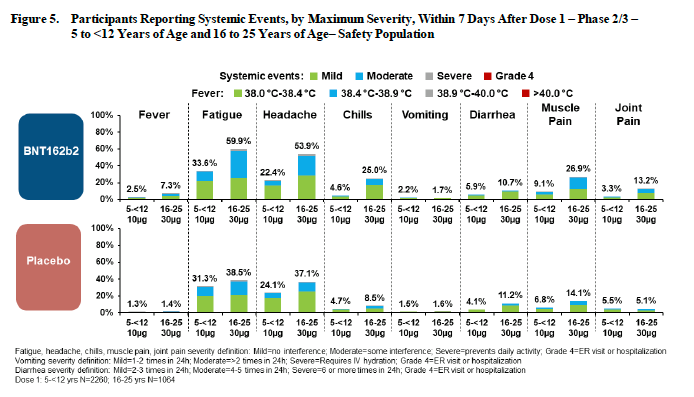
A total of 3 serious adverse events were reported during the trial (including foreign ingestion of a penny and a bone fracture), but deemed unrelated to the intervention 🤔.
NO cases of myocarditis or pericarditis were reported in the trial. While this is good news, given the relatively rare incidence of myocarditis reported in teens, statistically it’s not surprising to see no cases out of only 3000 vaccines given.
💡Effectiveness was measured in two ways:
➡️ “Immunobridging” analysis compared SARS-CoV-2 neutralizing antibodies in 5-11 year olds vaccine recipients to vaccinated 16-25 year olds (the group considered most clinically similar), finding similar neutralization ability in a lab dish. Neutralizing antibody activity was comparable against both the original “wild type” SARS-CoV-2 and the Delta variant.
➡️ Trial participants were followed up for confirmed symptomatic COVID-19 infection at least 7 days after their second dose. Vaccine efficacy was 💥90.7%💥, which included 3 cases in the vaccine group and 16 cases in the placebo group (with 2X as many vaccine as placebo participants). Note this trial was much smaller than the adult trials so being able to detect clear efficacy, even if the estimate is less precise, is notable. (See below for that now familiar but BEAUTIFUL vaccine efficacy figure).
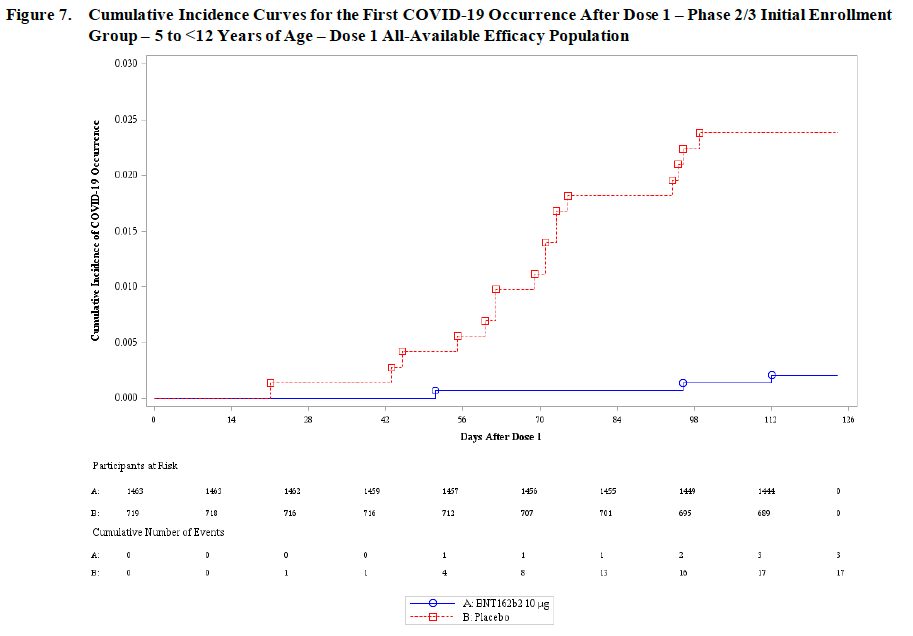
➡️ The FDA conducted their own analysis considering the potential risk of myocarditis versus the number of COVID-19 cases, hospitalizations and deaths prevented. We expect to hear more about this analysis during the meeting today, but the overall conclusion was in favor of vaccination except in scenarios of extremely low transmission. When other factors such as long COVID or educational disruptions are considered, the risk/benefit calculation in favor of vaccination becomes even stronger.
In other news, the U.S. is preparing the logistics for a vaccine roll-out for 5-11 year olds in the event approval comes AND Moderna also previewed positive clinical trial results for ages 6-11, stating that they will be submitting for approval to regulatory agencies soon.
💥 BOTTOM LINE💥:
Data from Pfizer/BioNTech show good safety and efficacy of the vaccine for 5-11-year-olds.
Based on the FDA’s own briefing document, support for recommending vaccination in this age group looks likely.
Stay tuned for more from the FDA and ACIP discussions!
For the SUPER NERDS out there, you can watch today’s meeting live.
Love,
Those Nerdy Girls
Links:
Full FDA documents:
Vaccines and Related Biological Products Advisory Committee October 26, 2021 Meeting Document
Tracking the FDA advisory panel meeting on Covid-19 vaccines for kids


