A: In simple terms, pool testing (also referred to as “group testing” or “batch testing”) combines many tests into one sample. The benefit is that it can facilitate an increase in testing capabilities as well as result in cost/resource savings (when supplies are low).
This is not a new strategy to test individuals for infectious diseases. The most famous example of pool testing comes from World War II (Syphilis in the U.S. military)…
The basics: Instead of testing many individuals, a pool is created with many individuals in one group. If the pool is positive, then additional tests are conducted to identify the individual who is positive. There are several methods to do this, with each having its advantages/disadvantages.
When pool testing works:
-When prevalence is low (to decrease risk of false negatives as well as to maximize testing resources)
-When used for population monitoring or “surveillance” (instead of only testing symptomatic individuals, it allows for testing of asymptomatic individuals with fewer resources utilized)
-When pools are of moderate size (if too big, evidence shows more test kits may be used)
International Growth Centre Article
-When complex methods are automated or a simple pooling strategy is used (if the pool model is complex such as those described here, it will be difficult for an individual in a lab to determine the testing sequence)
This is one option for rolling out testing. Last month, the FDA authorized Emergency Use Authorization (EUA). Early on, San Francisco and Nebraska used this method. Guidelines are also being developed now.
The bottom line: Pool testing could allow for more individuals to be tested. There are logistical issues that still may limit testing (such as supplies and standardization). Symptomatic testing of individuals will still be needed, but pool testing could be complementary.
Additional Articles for Background:
Pooled Testing Journal Article
~Aparna
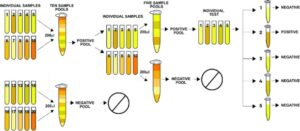
Photo credit: International Growth Centre


